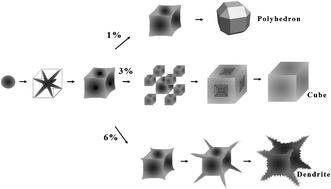
In this work, lanthanum hexaboride (LaB6) microcrystals were prepared by using the aluminum melt reaction method. The morphology evolution of LaB6 microcrystals was investigated by field-emission scanning electron microscopy (FESEM), and its growth mechanism was also discussed. As {111} planes have the highest growth rate, LaB6 crystals tend to form “perfect cubes” (equilibrium crystal morphology) with minimum total surface free energy. In Al melt with 3% of LaB6, the intrinsic preferential growth dominates and LaB6 microcrystals eventually grow into the “perfect cubes”, through a series of intermediate products, including “octa-pods” and “imperfect cubes”. By adjusting the concentration of reactants, polyhedral and dendritic LaB6 microcrystals were designed and prepared. The formation of these two non-equilibrium crystal morphologies is caused by a reduced growth rate of the preferential growth direction and the effect of constitution undercooling, respectively. Furthermore, the effect of LaB6 on the high temperature tensile strength of Al–Si alloy was also studied.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...