A dual-synthon in pyridinium chloride: formation of ladder-like and columnar motifs through hydrogen bonds and cation–π interactions†
Abstract
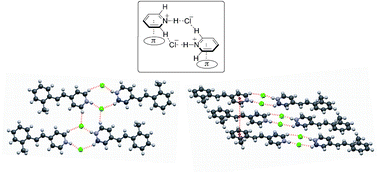
A supramolecular dual-synthon with 2R4(10) ring and columnar motifs is observed in crystal structures of all 2-, 3- and 4-methylstyrylpyridinium chlorides and 3-bromo-, 3-nitro- and 4-trifluoromethylstyrylpyridinium chlorides. The dual-synthon assembled through N–H⋯Cl− and C–H⋯Cl− hydrogen bonds and cation–π interactions forms both ladder-like and head-to-head or head-to-tail columnar motifs. The position of the methyl group at the benzene ring affects the size and shape of the channel structure. Infinite hydrogen bond networks involving chloride anions, water and HCl molecules are found in the channels of 3- and 4-methylstyrylpyridinium chlorides. Variations in electron-withdrawing groups such as Br, NO2 and CF3 have little effect on the crystal structures.

 Please wait while we load your content...
Please wait while we load your content...