Asymmetric domino synthesis of indanes bearing four contiguous stereocentres catalyzed by sub-mol% loadings of a squaramide in minutes†
Abstract
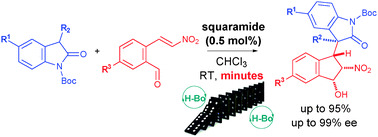
An efficient diastereo- and enantioselective synthesis of polyfunctionalized indanes bearing four contiguous stereogenic centres in generally very short reaction times and sub-mol% squaramide catalyst loadings has been developed. The novel methodology creates a maximum of two stereocentres per bond formation via an organocatalytic Michael–Henry domino reaction.

 Please wait while we load your content...
Please wait while we load your content...