Reduction of 2,2,2-trichloro-1-arylethanones by RMgX: mechanistic investigation and the synthesis of substituted α,α-dichloroketones†
Abstract
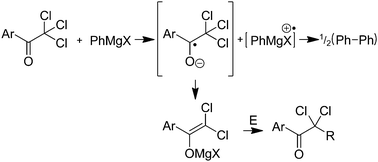
2,2,2-Trichloro-1-arylethanones undergo high yielding reductions to the corresponding 2,2-dichloro-1-arylethanones in the presence of RMgX. A single

 Please wait while we load your content...
Please wait while we load your content...