A 1,3-amino group migration route to form acrylamidines†
Abstract
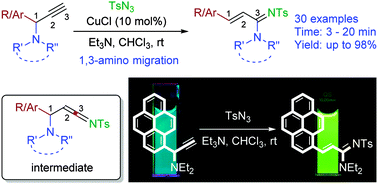
A novel 1,3-amino group migration strategy for the synthesis of acrylamidines is presented. Cu(I) catalyzed reaction of N,N-disubstituted propargylamine with tosylazide generates a highly reactive ketenimine intermediate which is trapped by a tethered amino group leading to the rearrangement reaction.

 Please wait while we load your content...
Please wait while we load your content...