Solvent effects on the molecular recognition of anions†
Abstract
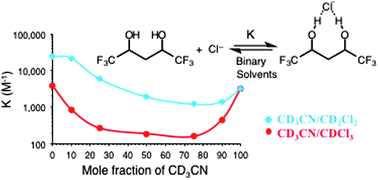
Anion recognition of two flexible diols in different solvents and binary mixtures were examined. Binding constants (K) in CD3CN and CDCl3 are surprisingly similar, and CD3CN–solvent mixtures led to reduced values of K that are smaller than in either pure solvent. A surprising U-shaped dependence is observed.

 Please wait while we load your content...
Please wait while we load your content...