Gated access to α-lithiated phenyltetrahydrofuran: functionalisation via direct lithiation of the parent oxygen heterocycle†‡
Abstract
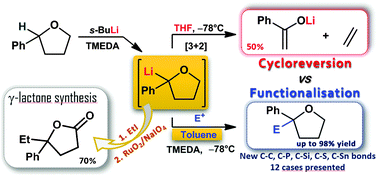
Cycloreversion of α-lithiated phenyltetrahydrofuran was successfully tamed at −78 °C in a non-coordinating solvent in the presence of TMEDA. This anion showed excellent nucleophilicity and could be intercepted with a variety of structurally different electrophiles to give 2,2-disubstituted derivatives which can be further elaborated to γ-butyrolactones.

 Please wait while we load your content...
Please wait while we load your content...