Asymmetric formation of tert-alkylamines from serinols by a dual function catalyst†
Abstract
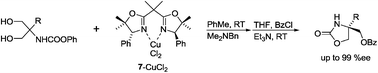
Consecutive intramolecular desymmetrization and kinetic resolution of 2-substituted N-phenoxycarbonylserinols have been achieved in one-pot by a single chiral catalyst, bisox(7)–CuCl2, to form oxazolidinones with remarkable enantioselectivities (94–99% ee) as tert-alkylamine building blocks. The process culminates in a dual-function of the chiral catalyst.

 Please wait while we load your content...
Please wait while we load your content...