Mechanochemical and silica gel-mediated formation of highly electron-poor 1-cyanocarbonylferrocene†
Abstract
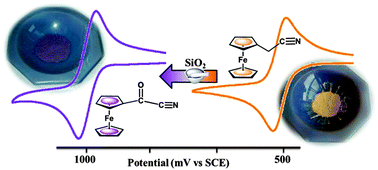
Simple manual grinding of solid cyanomethylferrrocene (1) and silica gel provides a facile one-pot access route to prepare unexpected and highly electron-poor metallocene, 1-cyanocarbonylferrocene (2). Electrochemical measurements supported by computational studies reveal that 2 exhibits the highest FeII/FeIII oxidation potential reported for mono-substituted ferrocenes.

 Please wait while we load your content...
Please wait while we load your content...