Abstract
A highly stereoselective rapid C-glycosylation reaction has been developed between glycal and unactivated alkynes in the presence of coppertriflate and ascorbic acid at low catalyst loading and at room temperature. A wide variety of glycals and aryl acetylenes participate in the reaction smoothly. TfOH generated during the reduction of Cu(OTf)2 by ascorbic acid may be the active catalyst for the glycosylation.
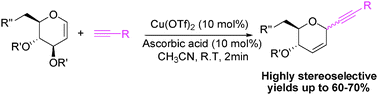

 Please wait while we load your content...
Please wait while we load your content...