Total synthesis of (−)-kaitocephalin†
Abstract
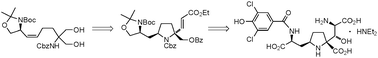
(−)-Kaitocephalin has been synthesized. With the C9 stereocenter from Garner's aldehyde, the C4 quaternary carbon was installed by the desymmetrization of the Cbz-protected serinol. The remaining stereogenic centers were generated through mercuriocyclization, epoxidation and regioselective epoxide opening, in which the quaternary carbon most likely played crucial roles in the stereoinduction.

 Please wait while we load your content...
Please wait while we load your content...