Iodine-mediated intramolecular amination of ketones: the synthesis of 2-acylindoles and 2-acylindolines by tuning N-protecting groups†
Abstract
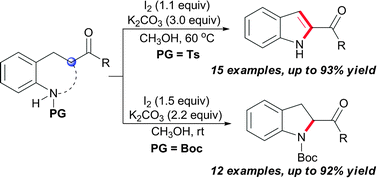
A general method for constructing both 2-acylindoles and 2-acylindolines via I2-mediated intramolecular C–N bond formation is presented, and the selective formation of either 2-acylindoles or 2-acylindolines just depends on the

 Please wait while we load your content...
Please wait while we load your content...