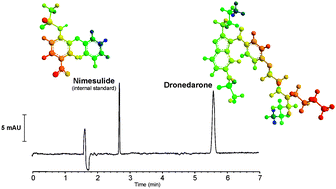
In this study, a micellar electrokinetic chromatography method was developed and validated for the analysis of dronedarone in film-coated tablets. Electrophoretic conditions were investigated by changing factors such as pH, buffer concentration, SDS concentration, capillary temperature, injection time and applied voltage. Separation was performed using a bare fused-silica capillary of 40.0 cm effective length (48.5 cm total length; 50 μm internal diameter) maintained at 30 °C and detection was set at 216 nm. Optimal conditions were obtained using 40 mM borate buffer and 50 mM SDS at pH 9.2 as running buffer with an applied voltage of 28 kV (positive polarity) and using hydrodynamic injection at 50 mbar for 7 s. The method was validated by evaluating typical validation characteristics such as specificity, linearity, accuracy, precision, the limit of detection, the limit of quantitation and robustness. The analytical curve was linear in the concentration range of 25 to 150 μg mL−1 (r = 0.9995). The accuracy was 99.9% and the relative standard deviations of repeatability and intermediate precision were lower than 2%. The limit of detection and limit of quantitation were 0.88 μg mL−1 and 2.66 μg mL−1, respectively. The method proved to be robust by a fractional factorial design evaluation. Forced degradation studies were performed by exposing dronedarone sample solution to stress conditions (acidic, basic, oxidative, thermal and photolytic) in order to verify the stability-indicating capability of the method. The MEKC method was successfully applied for the quality control of dronedarone hydrochloride in commercial film-coated tablets.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...