Electrochemical response of hydroxyurea by different voltammetric techniques at carbon paste electrode
Abstract
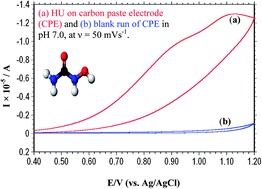
A simple and novel electroanalysis of hydroxyurea (HU) drug at carbon paste electrode has been investigated for the first time using cyclic, linear sweep and differential pulse voltammetric techniques. The oxidation of HU is irreversible and exhibits a diffusion controlled process and is pH dependent. The oxidation mechanism is proposed. The dependence of the current on pH, the concentration, nature of buffer, and scan rate is investigated to optimize the experimental conditions for the determination of HU. It was found that the optimum buffer for the determination of HU was pH 7.0, a physiological pH. In the range of 0.2 to 1.0 mM, the current measured by differential pulse voltammetry presents a good linear property as a function of the concentration of HU with a limit of detection of 6.52 μM. In addition, reproducibility, precision and accuracy of the method were checked as well. The developed method was successfully applied to HU determination in pharmaceutical formulation and human urine. The method finds its applications in quality control laboratories and pharmacokinetics.

 Please wait while we load your content...
Please wait while we load your content...