Development of purity certified reference materials for methanol, ethanol, acetonitrile, acetone, ethyl acetate and n-hexane by freezing point depression
Abstract
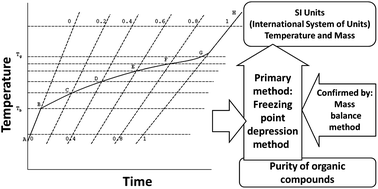
The accuracy of determination for commonly used organic compounds (such as methanol, ethanol, acetonitrile, acetone, ethyl acetate, and n-hexane) should be ensured by certified reference materials (CRMs), the values of which are traceable to SI units (International System of Units). Six new purity CRMs for these compounds were systematically studied and developed through qualitative analysis, quantitative analysis, confirmation, homogeneity study, stability study and evaluation of uncertainty. The method for quantitative analysis is a primary reference method (freezing point depression), which can establish the traceability between the purity values of the CRMs and the SI units of temperature and mass. The results of the primary reference method were confirmed by another method with a different principle (the mass balance method). The certified values of CRMs were (998 ± 2) mg g−1, (997 ± 2) mg g−1, (998 ± 2) mg g−1, (997 ± 3) mg g−1, (992 ± 4) mg g−1, (982 ± 4) mg g−1 (purity ± expanded uncertainty with confidence level of 95%) for acetonitrile, methanol, ethanol, ethyl acetate, acetone and n-hexane, respectively. Since the certified value was accurate, precise and traceable to SI units, these CRMs can ensure accuracy, comparability and traceability of results from different laboratories.

 Please wait while we load your content...
Please wait while we load your content...