Spectrophotometric determination of trace p-nitrophenol enriched by 1-hydroxy-2-naphthoic acid-modified nanometer TiO2 in water
Abstract
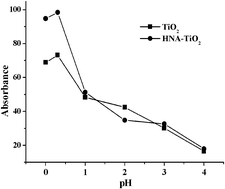
The surface of nanometer TiO2 was simply and quickly modified by chemical adsorption with 1-hydroxy-2-naphthoic acid (HNA), which can strengthen the affinity between TiO2 and p-nitrophenol (p-NP). A novel method for preconcentration of p-NP with HNA-modified nanometer TiO2 (HNA–TiO2) and determination by spectrophotometry has been developed. Under the optimum adsorption conditions, the best adsorption ratio of p-NP was greater than 98.0% at pH 0.3 after only stirring for 15 min, and the adsorptive capacity of HNA–TiO2 for p-NP was greater than 25.0 μg mg−1. The adsorbed p-NP could be eluted by 5 mL 2.0 mol L−1 NaOH and the elution percentage was greater than 92.0%. This method gave a concentration enrichment factor of 50 for a 250 mL sample, the detection limit (3σ, n = 11) was reduced to 25.4 μg L−1, the relative standard deviation was 3.10%. This simple and fast method was successfully applied to the determination of p-NP in industrial wastewaters and natural waters, so the interference from the matrix and similar organic pollutants (benzoate and phenol) could be avoided. Analytical recoveries of p-NP added to samples ranged from 93.5% to 104.1%.

 Please wait while we load your content...
Please wait while we load your content...