Isocratic RP-HPLC method for separation and simultaneous determination of ternary mixture of omeprazole, tinidazole and doxycycline in their raw materials and combined capsules
Abstract
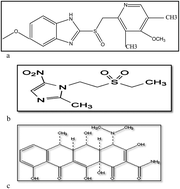
A simple, sensitive and rapid isocratic HPLC method has been developed for simultaneous estimation of a ternary mixture of omeprazole, tinidazole and doxycycline in both their raw state and combined in capsules, to be used for treatment of Helicobacter pylori . The analysis was performed on a C18 column (250 mm × 4.6 mm i.d., 5 μm particle size) with a mobile phase consisting of methanol and 0.06 M sodium dihydrogen phosphate (65 : 35, v/v) adjusted to pH 4.5. The UV detector was operated at 260 nm and the effluents were pumped with a flow rate of 1.0 mL min−1 using floctafenine as internal standard. The run time under the optimum chromatographic conditions is less than 6 min. Linearity, accuracy and precision were found to be acceptable over the concentration range of 2–20 μg mL−1 for omeprazole, 5–60 μg mL−1 for tinidazole and 5–30 μg mL−1 for doxycycline. The sensitivity of the method allows the determination of the studied drugs with a limit of quantification of 1.2 μg mL−1, 1.96 μg mL−1 and 1.88 μg mL−1 for omeprazole, tinidazole and doxycycline respectively. The proposed method was fully validated according to ICH guidelines. The high sensitivity and the simplicity of the proposed method allow the successful determination of such a ternary mixture in a combined capsule with a percentage recovery of 99.61% ± 0.79 for omeprazole, 99.69% ± 0.65 for tinidazole and 99.62% ± 0.94 for doxycycline.

 Please wait while we load your content...
Please wait while we load your content...