Nitrite determination in water samples based on a modified Griess reaction and central composite design
Abstract
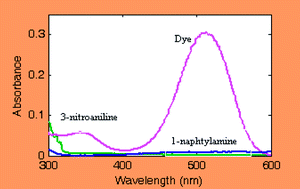
A simple, sensitive, selective and rapid spectrophotometric method for determination of trace amounts of nitrite was proposed. The method is based on the diazotization-coupling reaction of nitrite by 3-nitroaniline and 1-naphthylamine in hydrochloric acid solution at pH 1.0 (Griess reaction). The final product (an azo dye) has a purple color with maximum absorption at 515 nm. Optimum conditions of the variables influencing the reaction were explored by central composite design (CCD). The variables chosen were pH, reaction temperature, concentration of 3-nitroaniline and concentration of 1-naphthylamine. The univariate calibration curve was linear in the range of 0.01–1.70 mg L−1 of nitrite. A detection limit of 5 × 10−4 mg L−1 was obtained for the determination of nitrite by the proposed method. The modified Griess method was successfully applied to determine nitrite in tap, rain and wastewater samples. The results of the analysis of the real samples showed that percent relative standard deviation of the method (RSD%) is below 4% and percent recoveries vary between 99.0% and 102%.

 Please wait while we load your content...
Please wait while we load your content...