UV-vis spectrophotometric and artificial neural network for estimation of ammonia in aqueous environment using cobalt(ii) ions
Abstract
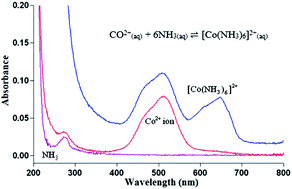
This paper reports the results for the quantitative determination of ammonia (NH3) in aqueous solution by a UV-vis spectrophotometric method and artificial neural network (ANN) intelligence tool. Quantitation of NH3 was based on the chemical reaction of NH3 with cobalt(II) (Co2+) ions in basic medium to form a blue hexamminecobaltate(II) ([Co(NH3)6]2+) complex. Characterizations of Co2+ ion in solution included photostability, pH effect, response time, Co2+ ion concentration effect, dynamic linear range and reproducibility, which were performed using a UV-vis spectrophotometer. The pink cobalt species gradually changed to blue with increasing NH3 concentration. The absorption calibration curve was linear over the NH3 concentration range of 0.6–3.5 mM at optimum pH 8 with a reproducibility relative standard deviation (RSD) of <4.0%. The interference effect was found to be negligible for a number of foreign ions present in the reaction medium during NH3 determination in an aqueous environment. A set of absorbance data for the [Co(NH3)6]2+ complex at selected wavelengths was input for ANN training using a back-propagation algorithm. The trained network with 22 hidden neurons, a 28 500 epoch number and 0.001% learning rate has extended the dynamic NH3 concentration range to 0.6–5.9 mM with a calibration error as low as 0.0649 × 10−3. The proposed ANN electronic sensor shows promise for NH3 estimation in unknown water samples based on pattern recognition.

 Please wait while we load your content...
Please wait while we load your content...