Effect of ammonium on liquid- and gas-phase protonation and deprotonation in electrospray ionization mass spectrometry†
Abstract
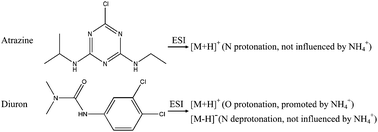
The electrospray ionization (ESI) is a complex process and there has been a long debate regarding the gas-phase effect on ion generation in the process. In this paper we investigated the effect of liquid chromatographic mobile phase additives (formic acid, aqueous ammonia and their combination) on the ESI signal intensities for a wide variety of compounds. The addition of a trace amount of aqueous ammonia to the common formic acid–methanol mobile phase significantly enhances the ESI signals of protonated molecules and suppresses the formation of sodium adduct ions. This effect is well observed for the compounds containing the –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group but not for those without N or O atoms. The ESI signal intensity of deprotonated molecules increases with increase in pH of the mobile phase for neutral compounds, such as substituted urea, whereas this trend is not observed for acidic compounds such as phenoxy acids. The mechanistic analysis regarding liquid- and gas-phase protonation and deprotonation is discussed.
O group but not for those without N or O atoms. The ESI signal intensity of deprotonated molecules increases with increase in pH of the mobile phase for neutral compounds, such as substituted urea, whereas this trend is not observed for acidic compounds such as phenoxy acids. The mechanistic analysis regarding liquid- and gas-phase protonation and deprotonation is discussed.

 Please wait while we load your content...
Please wait while we load your content...