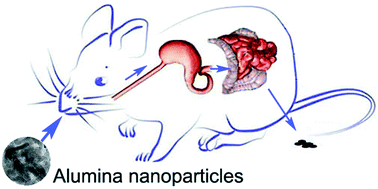
Bioavailability and preliminary toxicity evaluations of aluminananoparticlesin vivo after oral exposure
Abstract
* Corresponding authors
a College of Chemistry and Environment Protection Engineering, Southwest University for Nationalities, Chengdu 610041, China
b
Institute of Nanochemistry and Nanobiology, Shanghai University, Shanghai 200444, China
E-mail:
hwang@shu.edu.cn
Tel: +86-21-66138026
c Department of Clinical Laboratory, Third Hospital of Peking University, Beijing 100083, China
d Beijing National Laboratory for Molecular Sciences, Department of Chemical Biology, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
