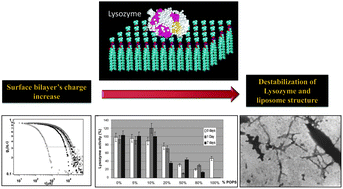
Hen egg-white lysozyme aggregates into amyloid-like assemblies when incubated at pH 2 and 60 °C for some days or under physiological conditions in the presence of lipid membranes. This makes the protein an ideal model to study the effect of membranes on the unfolding and aggregation of pathologically relevant peptides. In this study, by choosing appropriate ratios of phosphatidylserine and phosphatidylcholine, we modulate the surface density of negative charge to investigate the effects of lipid vesicles' composition on hen egg-white lysozyme aggregation. Aggregation was investigated with dynamic light scattering and zeta potential measurements; aggregate morphology was characterized by transmission electron microscopy. We found that negatively charged lipids are needed to allow protein–vesicle interaction; in addition, protein aggregation occurs only after a threshold of the liposomal surface charge density. The spectroscopic properties of two amyloid-specific fluorescent probes, Thioflavine T and Congo Red, provided information on the presence of an amyloid-like signature in the aggregates, and together with the determination of residual enzymatic activity, confirmed the importance of surface clusters of negative charge for lysozyme aggregation. Finally, the cytotoxicity data confirmed that a high content of acidic phospholipids in the vesicles is needed to induce growth of toxic aggregates.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...