Phase behaviour and complex coacervation of aqueous polypeptide solutions†
Abstract
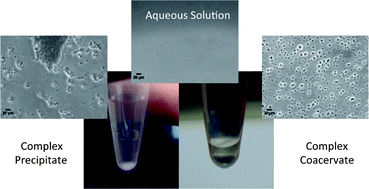
Mixing of oppositely charged polyelectrolytes in aqueous solutions may result in the formation of polyelectrolyte complexes (PEC). Phase separation and complex coacervation of polypeptides are investigated in this study. Polypeptides have identical backbones and differ only in their charged side groups, making them attractive model systems for this work. All experiments are conducted using equal chain lengths of polycation and polyanion in order to isolate and highlight effects of the interactions of the charged groups during complexation. Complex coacervation is strongly affected by the polypeptide mixing ratio (stoichiometry), ionic strength (salt concentration), total polymer concentration, pH, and temperature. To examine the effect of these parameters on complex formation we use sample turbidity as an indicator, and optical microscopy to discriminate between coacervate and precipitate. We establish phase diagrams as a function of polybase content and salt concentration using the critical salt concentrations required to reach the coacervate to solution boundary. Additionally, we examine the effect of molecular weight on the complex formation for the P(L-Lysine) (PLys)/P(L-Glutamic acid) (PGlu) system and establish a phase diagram. By determining the water content of the coacervate phase under various conditions we find that the salt content and stoichiometry of the mixed polyelectrolytes have a significant effect on the coacervate composition.
- This article is part of the themed collection: Polyelectrolytes in Soft Matter and Biology

 Please wait while we load your content...
Please wait while we load your content...