Structural insights into the potential of 4-fluoroproline to modulate biophysical properties of proteins†
Abstract
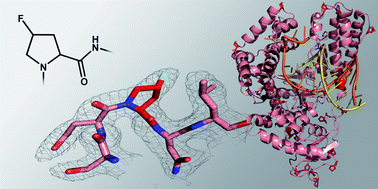
The unnatural amino acid 4-fluoroproline (4-FPro) can be used to replace natural proline in peptides and proteins to alter their stability, conformation and folding behavior. Interestingly, the two diastereomers (4R)- and (4S)-FPro behave quite differently resulting for example in increased or decreased protein stabilities. The reasons for the observed, opposed properties seem to be very complex and are not well understood yet, especially as only one single X-ray structure of a 4-FPro-modified protein is available, so far. The crystal structure of the large fragment of Taq DNA polymerase reported here far exceeds the molecular mass and number of 4-FPro residues of previous studied proteins and sheds light on how 4-FPro influences complex protein frameworks. It turns out that all aspects of prolyl Cγ-fluorination have to be considered in a combined fashion to understand how they account for the induced differences in protein stability. The interplay of different effects based on newly formed interactions and on the conformational preferences of 4-FPro determines whether the accepted diastereomer stabilizes or destabilizes the target protein. Due to counterbalanced effects, 4-FPro seems to be a very promising tool to even modify the properties of large enzymes with a high number of Pro residues since mono-fluorination at multiple sites is well tolerated by the target protein. Notably, the replacement of Pro by 4-FPro apparently also led to an improved crystallization capability of the DNA polymerase.

 Please wait while we load your content...
Please wait while we load your content...