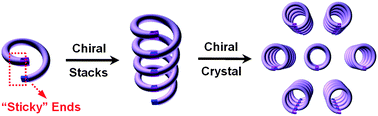
Biomolecules such as α-helices are exclusively right-handed without contamination by their left-handed counterparts. Within the abiotic world, chiral or external perturbations have to be applied to the abiotic helical foldamers or polymers to control and bias their helical screw sense. Otherwise, it is not possible to separate racemic helical foldamers composed of achiral building blocks. By designing two complementary “sticky” groups, incorporating them into the ends of helical pentamers and taking advantage of the energetically more favored full overlap, which involves helical backbones, we succeeded in demonstrating, for the first time, a spontaneous resolution of racemic helices into their enantiopure single-handed helical forms via chiral crystallization without the use of chiral auxiliary or external stimuli.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...