Complexes within complexes: hydrogen bonding in capsules†
Abstract
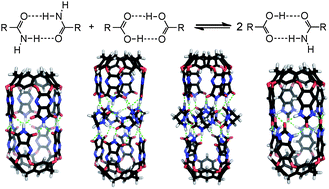
Hydrogen bonds play an important role in molecular recognition, biomolecule stabilization and organocatalysis. Typically, individual hydrogen bonding interactions are weak and short-lived in solution but isolated in capsules the encounters are intense and prolonged. We report here the interactions of

 Please wait while we load your content...
Please wait while we load your content...