Synthesis and enzymatic evaluation of ketose phosphonates: the interplay between mutarotation, monofluorination and acidity†
Abstract
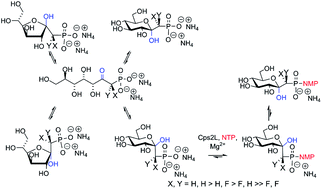
Ketose-phosphonates may adopt open chain, or α- or β-furanosyl, or α- or β-pyranosyl configurational isomers in aqueous solution. An HPLC and NMR analysis of a series of ketose-phosphonates with a thymidylyltransferase (dTDP-glucose pyrophosphorylase) implied a rapid dynamic equilibrium between the pyranosyl forms of gluco-ketose phosphonate leading to efficient production of unique sugar nucleotide analogues. The preparation of diastereomerically pure gluco-configured monofluoromethylenephosphonates enabled the determination of the thymidylyltransferase preference for CHF stereochemistry. The effects of acidity upon thymidylyltransferase substrate specificity were determined using a series of monofluoro- and difluoro- ketose-phosphonates. WaterLOGSY NMR spectroscopy demonstrated a switching of the ordered Bi-Bi mechanism with ketose-phosphonate substrates. Ketose-phosphonates are presented as a unique class of sugar 1-phosphate analogues with potential applications as glycosyltransferase probes.

 Please wait while we load your content...
Please wait while we load your content...