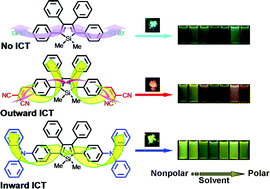
Three dimethyltetraphenylsiloles (DMTPSs) symmetrically substituted on their 2,5-positions with electron-accepting (A), i.e.aldehyde (ALD) and dicyanovinyl (DCV) or donating (D), i.e.diphenylamine (DPA) moieties were designed and synthesized via facile reaction procedures. The propeller-shaped luminogens exhibit aggregation-induced/enhanced emission characteristics with high quantum yields up to 74.0% in the solid state, and are thermally stable, showing high degradation temperatures and melting points up to 388 and 246 °C, respectively. Thanks to the contained A or D moieties, the siloles show intriguing solvatochromism: DMTPS-ALD exhibits almost no response to solvents due to the balance of electron affinities of the aldehyde and the silole core. Whereas, DMTPS-DCV and DMTPS-DPA possess outward intramolecular charge-transfer (ICT) from the silole core and the phenyl rings on its 3,4-positions to dicyanovinyl groups, and inward ICT from diphenylamine groups to the silole core, respectively, showing positive solvatochromism. A multilayer organic light-emitting diode using DMTPS-DPA among the luminogens as an emitter layer shows the highest performance with turn-on voltage, maximum luminance, current, power, and external efficiencies of 3.1 V, 13405 cd m−2, 8.28 cd A−1, 7.88 lm W−1, and 2.42%, respectively. Furthermore, DMTPS-DPA can also serve in hole-transporting layers because of its high hole-mobility. Therefore, the incorporation of a triphenylamine moiety into a silole system not only changes the classical aggregation-caused quenching fluorophore into AEE-active DMTPS-DPA, another example of “turning stone into gold”, but also enhances the hole-transporting ability of siloles.

 Please wait while we load your content...
Please wait while we load your content...