Selective sensing and efficient separation of Hg2+ from aqueous medium with a pyrene based amphiphilic ligand†
Abstract
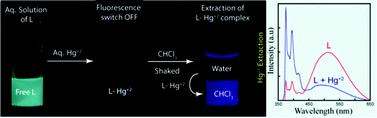
We have synthesized a new fluorogenic compound L, which can selectively bind and sense Hg2+ in aqueous medium over a broad pH range. It exhibits excellent selectivity for Hg2+ over a large number of competitive cations (Fe3+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Ag+, Cd2+, Pb2+, Ca2+, Mg2+, Na+, K+). The sensing ability of the ligand is studied by fluorescence and UV-vis spectroscopy. Hg2+ present even in a nanomolar range can be detected. The effectiveness of L for detecting Hg2+ inside live human cancer cells (HeLa) is also examined. The hydrophobic part of L is efficiently employed for the quantitative extraction of Hg2+ from an aqueous medium into the organic layer. The extraction ability of L is also estimated by NMR, fluorescence and atomic absorption spectroscopy showing that approximately 99% of the Hg2+ ions are extracted. These results imply that the compound has potential application for sensing and removal of Hg2+ ions from waste water in a large cross-section of mercury threatened zones around the world.

 Please wait while we load your content...
Please wait while we load your content...