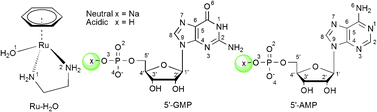
The thermodynamics and kinetics for the binding of the potential anticancer complex [(η6-benz)Ru(en)(H2O)]2+ (benz = benzene, en = ethylenediamine) (1) to nucleotides under neutral and acidic conditions were studied by density functional theory (DFT) calculations, employing 5′-guanosine monophosphate (5′-GMP) and 5′-adenosine monophosphate (5′-AMP) as model reactants. Based on the different binding modes, there were four pathways located for each nucleotide, two stepwise (a and b) and two concerted (c and d). In line with experiments, the reaction first proceeded with the binding of the 5′-phosphate group, and then underwent a slow intramolecular rearrangement to the N7 purine binding products. For 5′-GMP, concerted pathways are also possible based on our calculated results. The reaction of 5′-GMP is faster under acidic conditions than under neutral conditions. However, for the reaction of 5′-AMP, the first step of the phosphate binding is apparently more facile in a neutral environment than in an acidic one. Due to the thermodynamic sink of the phosphate bound intermediates, the second step of the intramolecular rearrangement from phosphate to A(N7)-binding exhibited a prohibitively high free energy of activation under neutral conditions. An approximately 9 kcal mol−1 difference in the reaction between 5′-GMP and 5′-AMP shows a clear preference for the binding of 5′-GMP over 5′-AMP, in agreement with experimental observation. It was also revealed that the phosphate as a hydrogen bond acceptor played an important role in the interaction of the Ru–arene complexes with nucleotides.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...