Particulate transepithelial drug carriers: barriers and functional polymers
Abstract
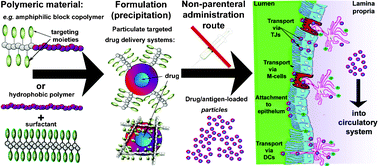
This review may serve as a compilation of helpful guidelines for design, synthesis, formulation, and in vitro, ex vivo as well as in vivo evaluation of particulate drug delivery systems that cross epithelial barriers. It provides both chemist and biologist with a comprehensive overview of complex anatomorphological features encountered by intranasally, pulmonary and orally administered particles. In addition, the materials (structures, modifications, formulations, and activity) used in transepithelial delivery are discussed. It covers the biological as well as physicochemical aspects crucial for the successful development of particulate drug carriers and methods for their application.

 Please wait while we load your content...
Please wait while we load your content...