Aromatization of IMDAF adducts in aqueous alkaline media†
Abstract
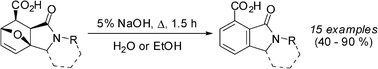
In this paper, we propose a simple synthesis of isoindoline-4-carboxylic acids by means of the aromatization of 3a,6-epoxyisoindoles in alkaline media. The method is facile from an experimental point of view: a short-term (0.5–2h) reflux of epoxyisoindoles in 5% aqueous solutions of alkali leads to the target products in 40–90% yields. The absence of by-products, ease of isolation of the target products and applicability to acidophobic group bearing substrates favorably distinguishes the proposed procedure from previously utilized acid-catalyzed methods. The proposed strategy has been successfully utilized for isoindole containing compounds and nuevamine-type

 Please wait while we load your content...
Please wait while we load your content...