Experimental and theoretical study of the degradation of malonamide extractant molecules under ionizing radiation†
Abstract
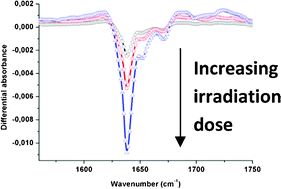
The behavior of three malonamides diluted in octane and submitted to ionizing radiation has been studied by means of in situ infrared spectroscopy, electrospray ionization mass spectrometry and quantum chemistry calculations. The major channel at low dose for a malonamide with ethyl groups on the nitrogen atoms and no substituent on the central carbon atom arises from the addition of an octyl group from the solvent. Adding alkyl chains on the nitrogen atoms and/or an alkyl chain on the central carbon atom weakens the central C–C bond which is then preferentially cleaved upon irradiation. The other bond cleavages (both C–N and C–H cleavages) are then detected when increasing the dose.

 Please wait while we load your content...
Please wait while we load your content...