Electrochemical hydrogen storage properties of a non-equilibrium Ti2Ni alloy†
Abstract
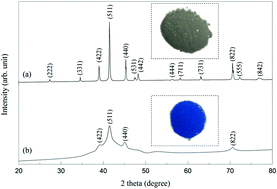
A crystalline Ti2Ni alloy was mechanically milled and subsequently annealed to form a non-equilibrium Ti2Ni alloy. This non-equilibrium structure could restrain the formation of irreversible

 Please wait while we load your content...
Please wait while we load your content...