A mechanistic study on the electrocatalysis of the Pu(iv)/Pu(iii) redox reaction at a platinum electrode modified with single-walled carbon nanotubes (SWCNTs) and polyaniline (PANI)†
Abstract
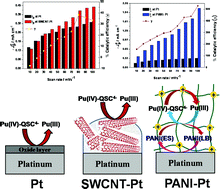
The electrochemistry of the Pu(IV)/Pu(III) couple in 1 M sulphuric acid solution was studied on bare and modified platinum electrodes by

 Please wait while we load your content...
Please wait while we load your content...