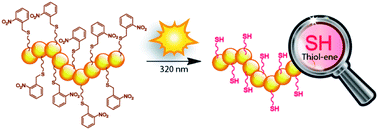
A novel and efficient methodology for the light-triggered release of thiols at ambient temperature is presented, which can be utilized for the in situ modification of polymeric backbones prepared via radical polymerization. Initially, a model reaction on poly(ethylene glycol) methyl ether was examined via size-exclusion chromatography coupled with electrospray ionization-mass spectrometry (SEC/ESI-MS) to establish the photodeprotection feasibility of 2-nitrobenzyl thioether moieties in the presence of variable activators or catalysts employed are Michael-type or radical thiol–ene chemistries, respectively. When 0.01 eq. of dimethylphenylphosphine is employed, disulfide coupling is reduced to its minimum and quantitative phototriggered formation of thiol-capped poly(ethylene glycol) methyl ether species is observed after a 16 hour irradiation period at 320 nm by a low-cost light source. The concept is extended to polymer backbone modification by atom transfer radical polymerization of the novel photosensitive monomer: 2-((3-((2-nitrobenzyl)thio)propanoyl)oxy)ethyl methacrylate containing the 2-nitrobenzyl thioether moiety. Well-defined homopolymers (4700 g·mol−1 ≤ Mn ≤ 20 000 g·mol−1, 1.29 ≤ PDI ≤ 1.40) containing one protected thiol per repeating unit were obtained and, upon a light stimulus (λmax = 320 nm), thiol entities are released along the lateral polymer chain. The photodeprotection process is mapped by exploiting the increased absorbance of photocleaved o-nitrosobenzaldehyde molecules at 345 nm and UV-Vis data suggests a quantitative backbone deprotection after a 16 hour irradiation time period. Further in situ functionalization of polymeric backbone is achieved via base-catalyzed maleimide–thiol addition at ambient temperature and its outcome is evidenced by a re-increased molecular weight in SEC, by virtue of decreased signal intensity of the 2-nitrobenzyl thioether moiety and the appearance of characteristic product protons in NMR spectroscopy (the polymer backbone functionalization is estimated as >90% by NMR analysis).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...