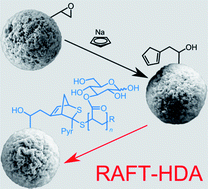
The facile and efficient functionalization of porous poly(glycidyl methacrylate) (pGMA) microspheres via hetero Diels–Alder (HDA) chemistry with poly(3-O-acryloyl-1,2:5,6-di-O-isopropylidene-α-D-glucofuranoside) (pAIpGlc) prepared by reversible addition–fragmentation chain transfer (RAFT) polymerization employing electron deficient thiocarbonylthio compounds (benzyl pyridin-2-yldithioformate (BPDF)) is described in detail. The efficiency of the employed ‘grafting to’ approach is qualitatively and quantitatively analyzed. Initially the microspheres are functionalized with a highly reactive diene – cyclopentadiene (Cp) – in one step with sodium cyclopentadienide, and subsequently reacted with a protected glycopolymer (number-average molecular weight, Mn = 4200 g mol−1; polydispersity index, PDI = 1.2) that carries a thiocarbonyl moiety functioning as a dienophile. The functionalization of the microspheres is achieved under mild conditions (T = 50 °C) with trifluoroacetic acid (TFA) as a readily removable catalyst. Deprotection of the grafted pAIpGlc to poly(3-O-acryloyl-α,β-D-glucopyranoside) (pAGlc) can be performed after functionalization in one pot with formic acid at ambient temperature. The obtained loading capacity is 2.63 × 1019 chains per g and the grafting density is close to 0.16 chains per nm2. Quantitative analysis of the grafting densities is achieved via elemental analysis; the pore size distribution before functionalization was analyzed by inverse size exclusion chromatography (iSEC). Further employed characterization techniques include scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS) and high resolution attenuated total reflectance (ATR) FT-IR microscopy supporting the successful modification of the microspheres.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...