Controlled folding of polystyrene single chains: design of asymmetric covalent bridges
Abstract
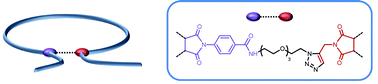
α-Shaped polystyrene origamis with variable sizes and loop diameters were synthesized using a simple folding strategy. Linear polystyrene precursors containing precisely incorporated reactive functions (i.e. alkyne and activated ester moieties) were first synthesized by sequence-controlled copolymerization of styrene (donor comonomer) with functional N-substituted maleimides (acceptor comonomers). These copolymers were prepared by nitroxide mediated polymerization in the presence of a commercially available alkoxyamine (i.e. BlocBuilder® MA). Discrete amounts (i.e. one molar equivalent as compared to the alkoxyamine) of triisopropylsilyl-protected N-propargyl maleimide and pentafluorophenyl 4-maleimidobenzoate were added at precise moments during the polymerization of a large excess of styrene. Due to the kinetically favored cross-propagation of donor and acceptor comonomers, the N-substituted maleimides were incorporated in precise regions of the polystyrene chains. Linear precursors with variable chain-lengths and sequence distributions were synthesized and characterized using 1H NMR, size exclusion chromatography (SEC) and FT-IR spectroscopy. Afterwards the chains were folded in solution using a two-step strategy. The pentafluorophenyl activated ester moieties were first reacted with 11-azido-3,6,9-trioxaundecan-1-amine, thus leading to azide-functionalized polymers. Subsequently, intramolecular azide–alkyne Huisgen cycloadditions were performed in dilute DMF solutions in the presence of a copper-based catalyst. SEC, 1H NMR, and FT-IR measurements indicated the formation of folded polymer chains containing asymmetric covalent bridges.
- This article is part of the themed collections: New methods of polymer synthesis and Celebrating The Five-Year Anniversary of Polymer Chemistry

 Please wait while we load your content...
Please wait while we load your content...