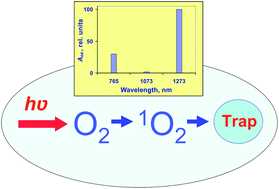
It is shown that the weak IR absorption bands corresponding to the forbidden triplet–singlet transitions in oxygen molecules can be reliably studied in air-saturated solvents under ambient conditions using measurements of the photooxygenation rates of singlet oxygen traps (1,3-diphenylisobenzofuran or uric acid) upon direct excitation of oxygen molecules by IR diode lasers. The best results were obtained from comparison of the oxygenation rates upon direct and photosensitized singlet oxygen excitation. In the present paper, this method was applied to estimation of the absorbance (Aox) and molar absorption coefficients (εox) corresponding to the oxygen absorption bands at 765 and 1273 nm in carbon tetrachloride, acetone, alcohols and water. In carbon tetrachloride, the band at 1073 nm was also investigated. Correlation of the obtained data with the luminescence spectra and radiative rate constants of singlet oxygen, contribution of oxygen dimols and biological significance of the studied effects are discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...