Photooxidative cleavage of zinc 20-substituted chlorophyll derivatives: conformationally P-helix-favored formation of regioselectively 19–20 opened linear tetrapyrroles†‡
Abstract
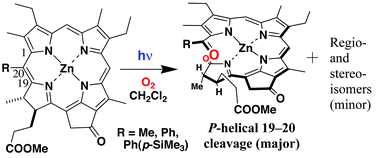
Photoreaction of zinc methyl 20-substituted meso(pyro)pheophorbide-a prepared by modifying naturally occurring chlorophyll-a in the presence of oxygen molecules gave its C19–C20
- This article is part of the themed collection: In honour of Professor Kurt Schaffner

 Please wait while we load your content...
Please wait while we load your content...