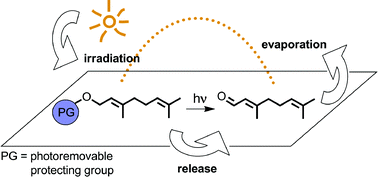
To develop their biological activity, bioactive volatile compounds, such as pheromones or fragrances, have to evaporate from surfaces. Because these surfaces are usually exposed to natural daylight, the preparation of non-volatile precursors using photoremovable protecting groups is an ideal tool to control the release of caged volatile molecules from various surfaces by light-induced covalent bond cleavage. Many photoreactions occur under mild environmental conditions and are highly selective. To break covalent bonds under typical application conditions, the photoreaction has to proceed at ambient daylight, to tolerate the presence of oxygen and to run in polar media (e.g. in water). The amount of volatiles generated from photochemical delivery systems depends on the light intensity to which the systems are exposed. Both photoisomerisations and photofragmentations have successfully been investigated for the slow release of caged pheromones and fragrances from their corresponding precursors.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...