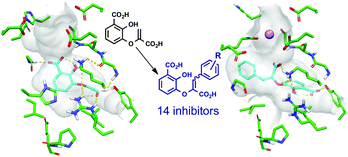
Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site†
Abstract
Mycobacterium tuberculosis salicylate synthase (MbtI) catalyses the first committed step in the biosynthesis of mycobactin T, an iron-chelating

 Please wait while we load your content...
Please wait while we load your content...