Mutual influence of backbone proline substitution and lipophilic tail character on the biological activity of simplified analogues of caspofungin†
Abstract
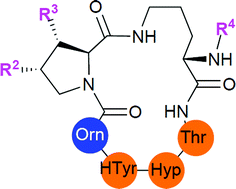
The echinocandins represent the most recent class of antifungal drugs. Previous structure–activity relationship studies on these lipopeptides have relied mainly upon semisynthetic derivatives due to their complex chemical structures. A successful strategy for the rapid enantioselective synthesis of the branched fatty acid chain of caspofungin and analogues was developed to synthesize several simplified analogues of caspofungin. The specific minimum inhibitory activity of each mimic was determined against a panel of Candida strains. This approach gave access to new fully synthetic derived caspofungin mimics with high and selective antifungal activities against Candida strains. In addition, the data suggested an important role of the hydroxy proline residue in the bioactive conformation of the macrocyclic peptide ring structure.

 Please wait while we load your content...
Please wait while we load your content...