Bioorthogonal metal-free click-ligation of cRGD-pentapeptide to alginate†
Abstract
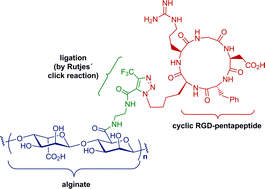
“Click” reactions have become very common and powerful ligation techniques, of which 1,3-dipolar cycloadditions have most frequently been employed. Since metal-mediated cycloadditions are incompatible in biomedical applications due to toxicity issues associated with transition metals like copper, metal-free variants provide important alternatives. The metal-free conjugation process is studied in detail with special emphasis put on the reaction progress. This report unfolds the first aqueous metal-free “click” conjugation of a cyclic RGD-pentapeptide with the biomacromolecule alginate, creating a “smart” bioactive polymer with potential applications in biomedicine.

 Please wait while we load your content...
Please wait while we load your content...