Substituent effects on axle binding in amidepseudorotaxanes: comparison of NMR titration and ITC data with DFT calculations†‡
Abstract
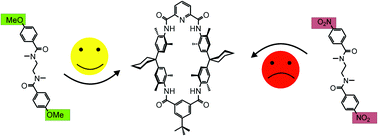
The binding behaviour of differently substituted diamide axle molecules to Hunter/Vögtle tetralactam
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...