Platinum catalyzed 7-endocyclization of internal alkynyl amides and its application to synthesis of the caprazamycin core†‡
Abstract
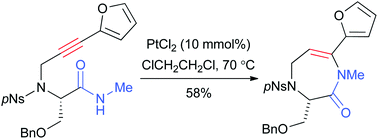
The scope and limitations of the platinum catalyzed 7-endo
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...