One-pot synthesis of 1-alkyl-1H-indazoles from 1,1-dialkylhydrazones viaaryne annulation†
Abstract
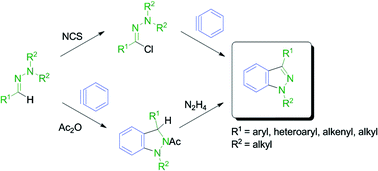
The reaction of readily accessible 1,1-dialkylhydrazones with commercially available o-(trimethylsilyl)aryl triflates provides a direct one-step route to pharmaceutically important 1-alkylindazoles. The products are obtained in high yields by one-pot NCS-chlorination/

 Please wait while we load your content...
Please wait while we load your content...