Visible light mediated homo- and heterocoupling of benzyl alcohols and benzyl amines on polycrystalline cadmium sulfide†
Abstract
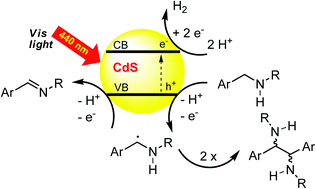
The oxidative coupling of sp3 hybridized carbon atoms by photocatalysis is a valuable synthetic method as stoichiometric oxidation reagents can be avoided and dihydrogen is the only byproduct of the reaction. Cadmium sulfide, a readily available semiconductor, was used as a visible light heterogeneous photocatalyst for the oxidative coupling of benzyl alcohols and benzyl amines by irradiation with blue light. Depending on the structure of the starting material, good to excellent yields of homocoupling products were obtained as mixtures of diastereomers. Cross-coupling between benzyl alcohols and benzyl amines gave product mixtures, but was selective for the coupling of tetrahydroisoquinolines to nitromethane. The results demonstrate that CdS is a suitable visible light photocatalyst for oxidative bond formation under anaerobic conditions.

 Please wait while we load your content...
Please wait while we load your content...