Total syntheses of pamamycin 607 and methyl nonactate: stereoselective cyclisation of homoallylic alcohols that had been prepared with remote stereocontrol using allylstannanes†
Abstract
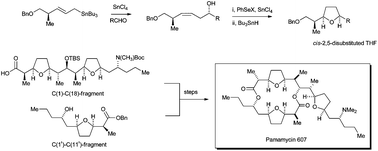
The tin(IV) chloride mediated cyclisation of (Z)-homoallylic alcohols using phenylselenenyl chloride or phthalimide in the presence of a Lewis acid followed by reductive removal of the phenylselenenyl group was found to give 2,5-cis-disubstituted tetrahydrofurans with excellent stereocontrol. Using this procedure, (2S,4S,8R,6Z)-9-benzyloxy-2-tert-butyldiphenylsilyloxy-8-methylnon-6-en-4-ol (11), prepared stereoselectively via the tin(IV) chloride promoted reaction between the (R)-5-benzyloxy-4-methylpent-2-enyl(tributyl)stannane (3) and (S)-3-tert-butyldiphenylsilyloxybutanal (10), gave (2S,3R,6S,8S)-1-benzyloxy-8-tert-butyldiphenylsilyloxy-3,6-epoxy-2-methylnonane (13) after deselenation. This tetrahydrofuran was selectively deprotected, oxidized and esterified to give methyl nonactate (2). Having established this synthesis of 2,5-cis-disubstituted tetrahydrofurans, it was applied to complete a synthesis of pamamycin 607 (1). (2S,3R,6S,8R)-1-Benzyloxy-8-[N-methyl-N-(toluene-4-sulfonyl)amino]-3,6-epoxy-2-methylundecane (35) was prepared stereoselectively from (R)-3-[N-(toluene-4-sulfonyl)-N-methylamino]hexanal (32) by reaction with the stannane 3 followed by cyclisation of the resulting alkenol 33 and deselenation. Following debenzylation and oxidation, an aldol reaction of the aldehyde 37 using the lithium enolate of 2,6-dimethylphenyl propanoate (61) gave mainly the 2,3-anti-3,4-syn-adduct 48. After protection of the secondary alcohol as its tert-butyldimethylsilyl ether 49, reduction using DIBAL-H and oxidation, the resulting aldehyde, (2S,3S,4R,5R,8S,10R)-3-tert-butyldimethylsilyloxy-2,4-dimethyl-5,8-epoxy-10-[N-methyl-N-(toluene-4-sulfonyl)amino]tridecanal (62), was taken through to the bis-tetrahydrofuran 65 by repeating the sequence of the reactions with the stannane 3, cyclisation and deselenation. The N-(toluene-4-sulfonyl) group was then replaced by an N-(tert-butoxycarbonyl) group and O-debenzylation and oxidation gave the carboxylic acid 70 that corresponds to the C(1)–C(18) fragment of pamamycin 607 (1). Similar chemistry was used to prepare the C(1′)–C(11′) fragment 89 of the pamamycin, except that in this case the configuration of the secondary alcohol introduced by the allylstannane reaction had to be inverted using a Mitsunobu reaction before the cyclisation. Esterification of the carboxylic acid of the C(1)–C(18)-fragment 70 using the alcohol 89 of the C(1′)–C(11′) fragment followed by selective deprotection, macrocyclisation, N-deprotection and N-methylation gave pamamycin 607 (1) that was identical to a sample of the natural product.

 Please wait while we load your content...
Please wait while we load your content...