Synthesis and properties of 1-(3′-dihydroxyboryl-2′,3′-dideoxyribosyl)pyrimidines†
Abstract
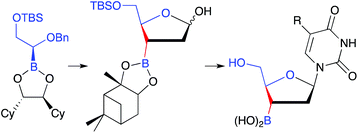
Nucleoside analogues having a boronic acid in place of the 3-hydroxyl group of deoxyribose have been synthesized. The synthesis of 3′-dihydroxyboryl-2′,3′-dideoxyribose was based on asymmetric homologation of boronic esters with (dihalomethyl)lithium, beginning from a (silyloxymethyl)boronic ester. A change of chiral director is required before introduction of the second stereocenter, and the direct displacement of (S,S)-1,2-dicyclohexyl-1,2-ethanediol by (1S,2S,3R,5S)-pinanediol was used for this purpose. Coupling of the pinanediol ester of the 1-acetoxy-3-dioxyboryl-5-tert-butylsilyloxy deoxyribose analogue with silylated pyrimidine bases was accomplished with trimethylsilyl bromide. The boronic acid nucleoside analogues were not cytotoxic toward Hep G2 (human hepatocarcinoma) cells. Decomposition occurred over a period of several hours at 37 °C, pH 7.4, with liberation of free pyrimidine base.

 Please wait while we load your content...
Please wait while we load your content...